The brand of collagen is critical @ . The recommended collagen hydrolysat in some studies is made by Nutri-Diem , distributed by Essentially Yours Industries @ , and marketed as a weight-loss supplement called Calorad @. You will notice a 20% chance of a miracle in these studies.
GOTO The Effects of Collagen Hydrolysat on Symptoms of Chronic
Fibromyalgia and Temporomandibular Joint Pain.
Gary B. Olson, D.D.S.; Sue Savage. M.B.S. JoAnn Olson, R.P.P.
The
Journal of Craniomandibular Practice 2000 Apr;18(2):135-41.
GOTO Collagen and muscle pathology in fibromyalgia
patients.
Gronemann ST, Ribel-Madsen S,
Bartels EM, Danneskiold-Samsoe B, Bliddal H.
The Parker Institute, Department
of Rheumatology, Frederiksberg Hospital, H:S University
Hospital.
GOTO Collagen cross-links in fibromyalgia syndrome
Sprott H, Muller A, Heine H
Clinical
Expermeriments in Rheumatology 1998 Sep-Oct;16(5):626-7.
GOTO Treatment of rheumatoid arthritis with oral type II collagen.
Results of a multicenter, double-blind, placebo-controlled trial.
Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D,
Weisman M, Fletcher MJ, Chasan-Taber S, Finger E, Morales A, Le CH, Trentham
DE.
Beth Israel Deaconess Medical Center, Boston, Massachusetts 02215, USA.
Arthritis Rheum. 1998 Feb;41(2):290-7.
GOTO Effects of oral administration of type II collagen on
rheumatoid arthritis.
Trentham DE,
Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, Hafler DA,
Weiner HL.
Department of Medicine, Beth Israel Hospital, Boston,
MA.
Science. 1993 Sep 24;261(5129):1727-30.
GOTO Oral type II collagen in the treatment of rheumatoid arthritis.
A six-month double blind placebo-controlled study.
Cazzola M, Antivalle M, Sarzi-Puttini P, Dell'Acqua D, Panni B, Caruso
I.
Rheumatology Unit, Azienda Ospedaliera Polo Universitario L. Sacco, Milan,
Italy.
Clin Exp Rheumatol. 2000 Sep-Oct;18(5):571-7.
GOTO Type II collagen serology: a guide to clinical responsiveness
to oral tolerance?
Gimsa U, Sieper J, Braun J,
Mitchison NA.
Deutsches Rheuma-Forschungs-Zentrum Berlin,
Germany.
Rheumatol Int. 1997;16(6):237-40.
GOTO Oral type II collagen treatment in early rheumatoid arthritis.
A double-blind, placebo-controlled, randomized trial.
Sieper J, Kary S, Sorensen H, Alten R, Eggens U, Huge W, Hiepe F, Kuhne
A, Listing J, Ulbrich N, Braun J, Zink A, Mitchison NA.
Deutsches Rheuma
Forschungszentrum, Berlin, Germany.
Arthritis Rheum. 1996
Jan;39(1):41-51.
GOTO Effects of oral administration of type II collagen on rheumatoid
arthritis.
Trentham DE, Dynesius-Trentham RA, Orav EJ,
Combitchi D, Lorenzo C, Sewell KL, Hafler DA, Weiner HL.
Department of
Medicine, Beth Israel Hospital, Boston, MA.
Science. 1993 Sep
24;261(5129):1727-30.
The Effects of Collagen
Hydrolysat on Symptoms of Chronic Fibromyalgia and Temporomandibular Joint
Pain
Gary B. Olson, D.D.S.; Sue Savage. M.B.S. JoAnn Olson, R.P.P.
The Journal of Craniomandibular Practice 2000 Apr;18(2):135-41.
ABSTRACT: Twenty (20) people who had medically diagnosed fibromyalgia for two to 15+ years participated in and completed a 90-day evaluation to determine effects of collagen hydrolysat on symptoms of chronic fibromyalgia, with twelve reporting temporomandibular joint pain. Collagen hydrolysat is a food supplement that is available without prescription, with no known side effects. Participants were evaluated initially and then at 30-, 60-, and 90-day periods. Final results were obtained and comparisons made. The average pain complaint levels decreased significantly in an overall group average, and dramatically with some individuals. It was concluded that patients with fibromyalgia and concurrent temporomandibular joint problems may gain symptomatic improvement in their chronic symptoms by taking collagen hydrolysat.
This study is a report of thirty (30) chronic fibromyalgia sufferers who were supplied collagen hydrolysat. Collagen hydrolysat is a food supplement made from a collagen protein formula which nutritionally feeds the body allowing it to support itself in maintaining lean muscle tissue. These individuals had tried various modalities of treatment to alleviate their symptoms and the reported levels of symptomatic complaints had plateaued with no further improvement despite repeated treatments. The symptomatic temporomandibular dysfunction (TMD) symptoms were treated without using a dental approach. Collagen hydrolysat helps the body to utilize fat and sugar effectively, as well as supports the body's natural mechanisms. A pain patient of Dr. Olson's reported that the Calorad (Nutr-Diem, Inc., Ste-Julie. Quebec, Canada) brand of collagen hydrolysat had effectively reduced her fibromyalgia symptoms, so the authors contacted the distributor, Essentially Yours Industries Corp (EYI). EYI referred them to the manufacturer, Nutri-Diem, Inc., and Medical Director, Jean-Louis Robillard, M.D., agreed to cooperate with the study by supplying product. The authors received no compensation of grant monies for this study.
Fibromyalgia Syndrome: Clinical Features
Fibromyalgia Syndrome (FMS) is
characterized by widespread pain, multiple tender points, fatigue, poor sleep,
feeling that extremities are swollen, and paresthesia. Data in recent years
suggest FMS is part of a spectrum of syndromes which have been termed
dysfunctional spectrum syndrome (DSS). Members of the DSS family include
irritable bowel syndrome, chronic fatigue syndrome, fibromyalgia,
temporomandibular dysfunction, tension-type headaches, migraine, and primary
dysmenorrhea, among others. The most important pathophysiological mechanisms
involved in DSS seem to be an aberration of neurohormonal functions. (1)
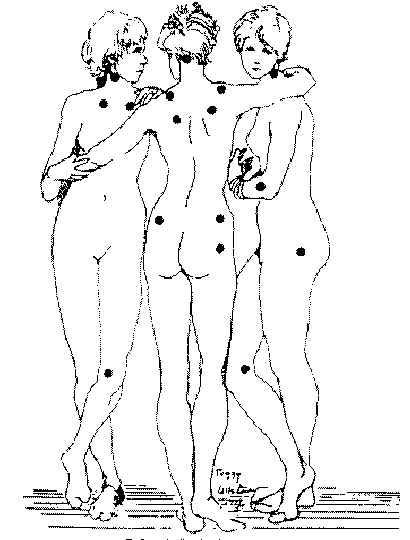
Widespread musculoskeletal pain along with multiple tender points form the core features of FMS. Widespread pain has been defined in the American College of Rheumatology (ACR) criteria for the classification of fibromyalgia as the presence of pain in the axial skeleton (cervical or thoracic or lumbar or anterior chest region plus above the waist and below the waist, plus right and left sides of the body). (2) The ACR criteria definition of widespread pain has sometimes been misinterpreted to mean pain in all four limbs as well as the spine, although such a wide distribution of pain is indeed commonly reported to clinical practitioners. (3) Pain is often regional initially and then spreads to multiple locations over months. Some patients may present with pain in one or two regions, such as the back, neck, or the chest area. Pain then becomes progressively widespread, severe, constant, nagging, and disabling in a majority of patients seen in a rheumatology clinic. Pain is the most common reason for consultation with a physician. Eighteen tender points were identified in the ACR criteria. Eleven of eighteen tender points satisfies tender point criteria ( Figure 1 ). (4)
Yunus and associates have demonstrated that the number of tender points in FMS is not influenced by the psychological status of the patients. It is now clear that there is a generalized decrease in pain threshold in FMS: the so called controlled points are significantly more tender in FMS than control groups. (2.5-7) Such global tenderness provides an important clue to the pathophysiological mechanisms in FMS and suggests that an aberration of central pain mechanisms involving neuroendocrine dysfunctions is most important when explaining the widespread pain and tenderness that exists in fibromyalgia. (3)
Sufferers of symptoms related to DSS syndromes can benefit from centrally
active drugs that modulate neurotransmitter or neurohormonal functions. Since it
is likely that multiple neurochemicals with complex and interacting roles are
operative in FMS, (8,9) it is not expected that the same
drug will work in all the syndromes or even in all patients with the same
syndrome. Wolfe, et al.(10) described the general
symptoms/associated symptoms of fibromyalagia.
Pain : The pain of FMS
has no boundaries. People describe the pain as deep muscular aching, burning,
throbbing, shooting and stabbing. Quite often, the pain and stiffness are worse
in the morning and you may hurt more in muscle groups that are used
repetitively.
Fatigue : This symptom can be mild in some patients and
yet incapacitating in others. The fatigue has been described as brain fatigue in
which patients feel totally drained of energy. Many patients depict this
situation by saying that they feel as though their arms and legs are tied to
concrete blocks, and they have difficulty concentrating.
Sleep disorder
: Most FMS patients have an associated sleep disorder called the alpha-EEG
anomaly. This condition was discovered in a sleep lab with the aid of a machine
which recorded the brain waves of patients during sleep. Researchers found that
FMS patients could fall asleep without much trouble, but their deep level (or
stage four) sleep was constantly interrupted by bursts of awakelike brain
activity. Patients appeared to spend the night with one foot in sleep and the
other one out of it. In most cases, a physician does not have to order expensive
sleep lab tests to determine if you have disturbed sleep. If you wake up feeling
as though you have just been run over by a Mack truck, what doctors refer to as
unrefreshed sleep, it is reasonable for your physician to assume that you have a
sleep disorder. It should be noted that most patients diagnosed with chronic
fatigue syndrome have the same alpha-EEG sleep patterns, and some FMS-diagnosed
patients have been found to have other sleep disorders, i.e., sleep apnea, sleep
myoclonus (nighttime jerking of the arms and legs), restless leg syndrome, and
bruxism (teeth grinding). The sleep pattern for clinically depressed patients is
distinctly different from that found in FMS of CFS.
Irritable Bowel
Syndrome : Constipation, diarrhea, frequent abdominal pain, abdominal gas, and
nausea represent symptoms frequently found in roughly 40-70 % of FMS patients.
Temporomandibular Joint Dysfunction (TMD) : This syndrome causes
tremendous face and head pain in one quarter of FMS patients. Most of the
symptoms associated with the condition are thought to be related to the muscles
and ligaments surrounding the joint and not necessarily the joint itself.
Chronic headaches : Recurrent migraine or tension-type headaches are
seen in about 50% of FMS patients and can pose a major problem in coping for
this group.

Figure 1 The eighteen tender points of the 1990 American College of Rheumatology criteria for the classification of fibromyalgia. Eleven of the 18 tender points satisfies the tenderness criteria. Points indicated on figure by black dots. (Figure used with permission: Lippincott, Williams & Wilkins, The Journal of Arthritis and Rheumatism , 1990; 33:160-172. more pictures
Other common symptoms : Premenstrual syndrome and painful periods, chest
pain, morning stiffness, cognitive or memory impairment, numbness and tingling
sensation, muscle twitching, irritable bladder, the feeling of swollen
extremities, skin sensitivities, dry eyes and mouth, frequent changes in eye
prescription, dizziness, and impaired coordination can occur.
Aggravating factors : Changes in weather, cold or drafty environments,
hormonal fluctuations (premenstrual/menopausal states), stress, depression,
anxiety, and over-exertion can all contribute to symptom flare-ups.3
Materials and Methods
Thirty (30) individuals
were selected on a first-come, first-serve basis after all members of the
Wisconsin Rapids Fibromyalgia/Chronic Fatigue Support Group were contacted by
mail. Based upon previous reports, the average FMS drop-off rate is 40% in
studies. (10) The authors selected thirty people for this
pilot study, so even if a 40% drop-off occurred, we would still have beneficial
data to evaluate. None of the participants were in treatment for TMD problems at
this office. Requirements for participation in the study were:
1. Must be diagnosed with fibromyalgia by a physician for at least one year;
2. No previous/current use of collagen hydrolysat products;
3. Complete
medical history, fibromyalgia symptom history, and periodic updates;
4.
Obtain permission from a medical doctor, if necessary, to participate if
questions arose regarding other medications/medical conditions;
5. Subjects
were given information and understood that collagen hydrolysat is a food
supplement with no known side effects.
Calorad (Nutri-Diem, Inc., Ste-Julie, Quebec, Canada)
Fibromyalgia Research Project
Beginning of Study/End of Study
| Patient | Pain Level | Fatigue | Total Sleep | Uninterrupted Sleep | Irritable Bowel | Chronic Headache | TMD | Morning Stiffness | Cognitive Memory Impairment | Numbness/ Tingling Sensations | Feeling of Swollen Extremities | |||||||||||
| beg | end | beg | end | beg | end | beg | end | beg | end | beg | end | beg | end | beg | end | beg | end | beg | end | beg | end | |
| 1 | 4-9 | 2-5 | 50% | 50% | 7 | 8 | 4 | 5 | 6 | 6 | 6 | 4 | 8 | 5 | 10 | 8 | 5 | 3 | 2 | 5 | 4 | 4 |
| 2 | 2-3 | 2-3 | 75% | 75% | 6 | 7 | 4 | 4 | 4 | 2 | 7 | 3 | 2 | 1 | 1 | 1 | 1 | 2 | 9 | 3 | 5 | 1 |
| 3 | 6-7 | 6-7 | 75% | 75% | 7 | 7 | 2 | 3 | 3 | 6 | 7 | 5 | 1 | 1 | 7 | 3 | 1 | 1 | 9 | 8 | 1 | 1 |
| 4 | 8-9 | 2-3 | 75% | 50% | 12 | 8 | 4 | 6 | 10 | 4 | 10 | 4 | 10 | 4 | 10 | 6 | 10 | 4 | 10 | 2 | 10 | 2 |
| 5 | 6-7 | 2-3 | 75% | 75% | 6 | 8 | 4 | 6 | 9 | 4 | 1 | 1 | 1 | 1 | 8 | 3 | 7 | 3 | 7 | 3 | 10 | 10 |
| 6 | 4-5 | 4-5 | 50% | 55% | 6 | 8 | 2 | 3 | 8 | 5 | 10 | 5 | 1 | 1 | 8 | 5 | 5 | 5 | 5 | 8 | 8 | 8 |
| 7 | 4-5 | 4-5 | 25% | 55% | 12 | 10 | 4 | 4 | 5 | 4 | 5 | 4 | 1 | 1 | 8 | 5 | 5 | 5 | 8 | 4 | 1 | 5 |
| 8 | 8-10 | 6-7 | 75% | 50% | 5 | 5 | 3 | 3 | 6 | 4 | 10 | 9 | 9 | 5 | 9 | 7 | 8 | 3 | 8 | 6 | 7 | 5 |
| 9 | 6-7 | 2-3 | 50% | 25% | 7 | 4 | 3 | 4 | 2 | 1 | 1 | 1 | 1 | 4 | 4 | 1 | 2 | 1 | 10 | 1 | 2 | 3 |
| 10 | 4-5 | 2-3 | 50% | 75% | 7 | 7 | 4 | 5 | 7 | 2 | 3 | 2 | 6 | 1 | 9 | 4 | 4 | 2 | 9 | 4 | 7 | 1 |
| 11 | 6-10 | 9-10 | 0% | 0% | 6 | 5 | 3 | 3 | 5 | 2 | 10 | 4 | 10 | 4 | 7 | 4 | 7 | 2 | 1 | 1 | 2 | 1 |
| 12 | 4-5 | 4-5 | 25% | 50% | 9 | 6 | 3 | 5 | 4 | 3 | 5 | 5 | 6 | 3 | 9 | 8 | 9 | 7 | 2 | 3 | 2 | 2 |
| 13 | 4-5 | 4-5 | 75% | 50% | 7 | 5 | 3 | 2 | 6 | 2 | 7 | 4 | 4 | 2 | 9 | 6 | 8 | 6 | 7 | 4 | 9 | 5 |
| 14 | 2-3 | 2-3 | 50% | 75% | 9 | 9 | 5 | 5 | 4 | 2 | 2 | 2 | 1 | 1 | 8 | 7 | 5 | 3 | 4 | 4 | 4 | 1 |
| 15 | 4-5 | 4-5 | 50% | 25% | 7 | 8 | 5 | 5 | 6 | 6 | 2 | 2 | 5 | 2 | 8 | 10 | 9 | 4 | 4 | 2 | 2 | 1 |
| 16 | 8-10 | 8-10 | 50% | 50% | 8 | 9 | 4 | 4 | 2 | 6 | 4 | 1 | 1 | 6 | 10 | 10 | 7 | 9 | 8 | 6 | 5 | 9 |
| 17 | 4-5 | 4-5 | 50% | 75% | 6 | 5 | 3 | 5 | 10 | 4 | 5 | 5 | 1 | 1 | 10 | 4 | 10 | 3 | 10 | 1 | 3 | 2 |
| 18 | 6-9 | 6-9 | 75% | 25% | 6 | 5 | 3 | 2 | 8 | 7 | 8 | 10 | 5 | 1 | 10 | 10 | 8 | 10 | 8 | 7 | 8 | 7 |
| 19 | 4-7 | 4-7 | 25% | 75% | 6 | 7 | 2 | 7 | 9 | 3 | 5 | 3 | 1 | 1 | 10 | 3 | 7 | 3 | 8 | 2 | 9 | 3 |
| 20 | 10 | 10 | 0% | 50% | 10 | 6 | 2 | 3 | 2 | 1 | 10 | 4 | 1 | 1 | 10 | 7 | 10 | 7 | 10 | 4 | 1 | 3 |
Pain levels based on a scale of 0-10; 0-1=no pain; 2-3=mild
pain; 4-5=discomforting pain; 6-7=distressing pain; 8-9=intense pain;
10=excruciating pain
Fatigue based on percentage of normal, normal=100% and
complete exhaustion=0%
Patient #10 was the only male test patient.
Note;
non-pain symptoms based on a scale of 0-10 with 10 being worst and 0-1 being
absent.
The study participants were given a 30-day supply of the
Calorad (Nutri-Diem. Inc., Ste-Julie, Quebec, Canada) brand of collagen
hydrolysat and instructed to take one tablespoon with a glass of water just
before going to sleep. They were advised to take no food or other beverages
except water for three hours before taking the supplement. After 30 days they
returned to fill out a symptomatic update form and receive a second 30-day
supply. After 60 days another symptom report was obtained and another 30-day
supply given. At the end of 90 days, a final survey form was completed. Patients
were not coached. No controls were used in this initial investigative study. The
ages of the participants ranged from 31 to 75 years old. Their duration of
illness was 2 to 15 years, with the average being 5.35 years.
Previous modalities tried by the participants included: varied pillows, 8; medications, 18; food supplements, 10; exercise, 20; newsletters, 8; counseling, 5; an active support group, 12; herbal supplements, 13; physical therapy/ chiropractic care/massage, 16; acupuncture, 1.
Results
One male and nineteen females completed the study. All reported data is based upon this group of 20. Thirty people began the study and only three were noncompliant. The others had to stop during the study because: couldn't sleep, moved, home burned down, medically too ill, assault, and hospitalized. Twenty participants completed the 90-day study and the results follow. Table 1 shows both the beginning and end of study symptomatic responses. The only side effects reported were due to not eating and stomach jumpiness or loose stools, attributable to continued problems with hyper-irritable bowel. The people needing food beforehand were advised to have up to one cup of fresh fruit up to one hour before taking collagen hydrolysat, and those with loose stools were told to cut their dosage to one teaspoon and increase from there to the one tablespoon dosage per Nutri-Diem recommendation. No other side effects were reported.
Participants were instructed to change no current medications, food, or activities. The only change incorporated into their daily life was the addition of the collagen hydrolysat food supplement at bedtime as directed.
Discussion
In general, collagen is the building block of the soft tissues in the body. These tissues include all of the organs, skin, arteries, veins, lymph vessels, joint cartilage, and all of the muscles of the body. We are constantly involved in a catch up game to do repairs. Through the years, we lose lean muscle mass, have less demand for energy, and our metabolism adjusts and decreases. Therefore, it follows that providing the body with the collagen it needs for repairs and growth may help deficient areas to improve health.
According to Tim O'Shea, D.C., from California, a nutrition expert and chiropractor, it has been postulated that when the Calorad brand of collagen hydrolysat is absorbed on an empty stomach, it avoids anabolic competition from other amino acids, fatty acids, monosaccharides, sugars, and other absorbable elements. The concentrated effect of select amino acids in the blood-stream allows targeting of specific organs, in this case, the endocrine system. Calorad (Nutri-Diem, Inc., Ste-Julie, Quebec, Canada) has a specific amino acid formulation that targets the production of new collagen and thus supports natural function by providing in abundance long chain and branched chain amino acids necessary for connective tissue production (personal communication dated 8/18/99).
The proprietary formulation differs from other collagen supplements which are
made from hydrolyzed collagen, not collagen hydrolysat. Collagen hydrolysat has
a longer peptide chain composition (personal communication dated 1/24/00 from M.
Hittner, Family Natural Foods, Wisconsin Rapids, WI).
This study used
numerical symptomatic reporting of evaluated symptoms. When a patient goes to
his/her doctor, 90% of treatment is based upon symptomatic complaints. Results
reported are symptom changes reported and do not address personal or other
medical conditions that could also affect responses. These aggravating factors
could involve symptoms with emotional factors and chronic pain, medical
illnesses that can be co-morbid symptom contributors, menstrual cycle effects,
as well as changes in weather that may be occurring at the time of reporting.
Most studies of medication/treatment trials do not state if the evaluations were
done in the winter or summer months. Fibromyalgia sufferers are worse in the
winter and better in the summer. (10) This study was done
during the period January to April, 1999. Also important is the fact that over
50% of the participants in the study were taking other prescription medications
and over-the-counter drugs. Yet, it is important to also remember that these
fibromyalgia syndrome sufferers had experienced reported symptoms an average of
5.35 years, so any notable improvement is significant to report.
Sleep reporting in research studies is at times questionable. However, the presence of alpha EEG sleep anomaly has been interpreted as an arousal or a physiologic correlate of the complaint of light and un-refreshing sleep; an index of a non-restorative sleep. Consistent with this notion, fibromyalgia patients undergoing treatment showed significant correlations of symptoms with measures of sleep alpha EEG. Furthermore, in a recent study, fibromyalgia patients showed more alpha EEG sleep than healthy controls. (11)
In addition, a greater number of pre- and post-sleep pain patients were more likely to rate themselves as not having enough sleep, feeling less rested with more fatigue and sleepiness than the control subjects. While fibromyalgia patients did not indicate more vigilance during their sleep, they were better able to:
Recall in the morning their awake behavioral signaling; and
Estimate
their total time asleep as measured by their sleep EEG. (11)
Based upon this information, the authors were confident using self-reporting
assessment of sleep changes.
In spite of those possible neutralizing factors, very noticeable changes were noted. At first glance, the results ( Table 1 ) were varied. Some people improved dramatically while others did not change. The most notable average improvement ( Table 2 ) was a 25% decrease in overall reported fibromyalgia pain. Also observed was improvement in non-interrupted sleep from 3.35 hours to 4.2 hours. This is a 25% improvement and allowed deeper sleep to approach more restful sleep levels.
Further review of Table 2 reveals a 36% overall improvement in irritable bowel syndrome and 34% improvement in chronic headaches. Morning stiffness improved by 32% and cognitive/memory impairment improved 35%. Other average changes showed an improvement of 11% in numbness/tingling sensations. TMJ average change was 39% improvement. Reported fatigue levels did not change beyond a 2.5% improvement.
More in-depth review of Table 1 shows different results, however, in regard to TMJ problems. Eight people did not have TMJ complaints to start. Of the twelve who did have TMJ complaints, two did not improve and actually worsened. Ten participants improved noticeably. The interesting positive results regarding TMJ complaints thus reveals that the collagen hydrolysat appears to be a beneficial food supplement for reducing TMJ problems. None of the people received TMJ treatment via routine dental modalities of care. The authors postulate that changes in sleep and a decrease in muscle and ligament pain provided symptomatic relief to TMJ discomfort by decreasing overall body stress, similar to the overall adaptation/accommodation breakdown reported by Fonder and Sleye. (12)
Table 1 draws attention to participants #4, #5, #10, #17, and #19. These five people had remarkable improvement reports in most areas including sleep. This fact brings the authors to a summary of results that each person's response to collagen hydrolysat is based upon the individual experience, and closer scrutiny of Table 1 shows signifcant changes occurred. Our interpretation of the study results is that given the skeptical attitude of these FMS patients, plus the longstanding, well-documented nature of their disorder, we concluded the reported improvements are unlikely to be attributable to an expectancy effect.
Calorad (Nutri-Diem, Inc., Ste-Julie, Quebec, Canada) Fibromyalgia Research Project Group Averages*
| Beginning | 30 days | 60 days | 90 days | Ave. chg. | Results | |
| Pain level (based on 0-10 scale)** | 6.8 | 5.8 | 4.85 | 5.1 | -1.70 | 25% decrease |
| Fatigue | 50% | 48.75% | 51.25% | 52.5% | -2.50% | 2.5% decrease |
| Total Sleep | 7.45 | 6.9 | 7.4 | 7.1 | -0.35 | 0.5% hr. decrease |
| Uninterrupted sleep | 3.35 | 3.95 | 4.1 | 4.2 | 0.85 | 25% deeper sleep |
| Irritable bowel | 5.8 | 5.25 | 4.25 | 3.7 | -2.10 | 36% improvement |
| Chronic headache | 5.9 | 4.95 | 4.5 | 3.9 | -2.00 | 34% improvement |
| TMD (jaw) muscles & ligaments | 3.75 | 3.65 | 2.5 | 2.3 | -1.45 | 39% improvement |
| Morning stiffness | 8.25 | 6.45 | 5.9 | 5.6 | -2.65 | 32% improvement |
| Cognitive/memory impairment | 6.4 | 5.0 | 4.4 | 4.15 | -2.25 | 35% improvement |
| Numbness/tingling sensations | 6.95 | 4.6 | 3.7 | 3.9 | -3.05 | 44% improvement |
| Feeling of swollen extremities | 5.0 | 4.1 | 3.6 | 3.7 | -1.30 | 26% improvement |
* When more than one number was given, the higher number was
always used for averaging.
** Pain levels based on a scale of 0-10: 0-1 = no
pain; 2-3 = mild pain; 4-5 = discomforting pain; 6-7 = distressing pain; 9-9 =
intesnse pain; 10 = excruciating pain.
Note: Nonpain symptoms based on a
scale of 0-10 with 10 being worst and 0-1 being absent
Conclusions
In this pilot study, 20 participants took the Calorad (Nutri-Diem. Inc.,
Ste-Julie. Quebec, Canada) brand of collagen hydrolysat at bedtime for 90 days.
Review of the averaged data shows that this food supplement provided notable
improvement in symptomatic complaints. When reviewing individual responses
rather than those of the averaged group, very significant changes occurred. TMJ
complaints were notably improved in those who had been diagnosed with TMJ
problems.
The results of this preliminary study of a chronic fibromyalgia population are promising. These participants had tried many other modalities of treatment and were stuck with little hope of further improvement. However, study results have shown that a food supplement may provide notable improvement. These results show that further objective collagen hydrolysat studies need to be conducted, perhaps under a more controlled approach utilizing outcome measures which include the Fibromyalgia Impact Questionnaire, Self-Efficacy Questionnaire, and other more detailed tests. (13) Further research may show what effect this food supplement provided to stimulate improvement in a fibromyalgia population who had little hope of improvement.
The authors postulate that collagen hydrolysat may be one of the
neurohormonal protein/amino acid complexes necessary for balanced neuroendocrine
function. This mechanism of individual protein keys has already been proven by
Dr. Gunter Blobel, the 1999 Nobel Prize in Medicine winner. (14) His work over three decades has proven that proteins become
building blocks for the cells, while others function as enzymes catalyzing
thousands of chemical reactions as specific as ZIP codes, helping them find
their correct locations within the cells and providing normal function.
References
1. Reynolds WJ., Chiu B., Irman RD: Plasma substance P levels in
fibrositis. J. Rheumatol 1988; 15:1802-1803
2. Russel IJ., Vaeroy H., Javors
M., Nyberg F: Cerebrospinal fluid biogenic amine metabolites in
fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum 1992;
35:550-556.
3. Littlejohn GO., Weinstein C., Helme RD: Increased neurogenic
inflammation in fibrositis syndrome. J Rheumatol 1997; 14:1022- 1025.
4.
Wolfe F., Smythe. HA.,Yunus MB, et al.: the American College of Rheumatology
1990 criteria for the classification of fibromyalgia: report of the Multicenter
Criteria Committee. J Arthritis Rheum 1990; 33:160-172.
5. Houvenagel E.,
Forzy G., Cortet B., Vincent G: 5-Hydroxy indol acetic acid in cerebrospinal
fluid in fibromyalgia, Arthritis Rheum 1990; 33:555.
6. Russell IJ:
Neurohormonal aspects of the fibromyalgia syndrome. Rheum Dis Clin North Am
1989; 15:149-168
7. Yunus MB., Dailey JW., Aldag JC., Masi AT., Jobe PC:
Plasma tryptophan and other amino acids in primary fibromyalgia- a controlled
study. J Rheumatol 1992; 19:90-94
8. Wolfe F., et al: The American College
of Rheumatology 1990 criteria for the classification of fibromyalgia: report of
the Multicenter Criteria Committee. Arthritis Rheum 1990; 33:160-172
9.
Hudson JI., Hudson MS., Pilner LF., Goldenberg DL., Pope HGJ: Fibromyalgia and
major affective disorder: a controlled phenomenology and family history study.
Am J Psychiatry 1985; 142:441-446.
10. Goldenberg DL: Medications clinical
trials in fibromyalgia. J Musc Pain 1994; 2(3):142.
11. Lue F:
Neuroendocrine and metabolic aspects of fibromyalgia. J. Musc Pain 1994;
2(3):91.
12. Fonder A: The dental physician. Rock Falls, IL: Medical-Dental
Arts : VIII-XV, 1985.
13. Crofford L: Neuroendocrine aspects of
fibromyalgia. J. Musc Pain 1999; 7(4):129.
14. Altman L: Molecular biologist
wins Nobel Prize in medicine. New York Times . October 12, 1999.
Ms. Sue Savage, M.B.S., received her Medical Business Specialist certification from the State Medical Society of Wisconsin in 1996. She is the Administrative Assistant to Dr. Gary Olson and provides nutritional counseling to pain patients. She has used her college background to establish her own business, Healthy Concepts, based upon her special interest in natural products for healthy living. At Healthy Concepts she also provides nutritional counseling, aromatherapy, and is an herbal body wrap technician, as well as a certified instructor for the Herbal Body Wrap. Inc.
Ms. JoAnn Olson , R.P.P., is a Registered Polarity Practitioner, Cranio Sacral Therapist, and Reiki Master. She practices and teaches at Whole Person Unlimited, her private practice in Mosinee, Wisconsin. For the past 25 years she has pursued studies in the natural health field. She is certified in Herbology, Iridology, Bio-Mechanics, Muscle Response testing, Foot Reflexology, and Touch for Health. Other studies include acupressure, shiatsu, aromatherapy, yoga, therapeutic touch, and fascial tissue unwinding. She studied with renowned authors Bernie Siegel. M.D., author Love, Medicines & Miracles, Dr. Alan Siegel, author of Polarity Therapy, The Power that Heals, and Live Food Therapy with Dr. Ann Wigmore. Professional memberships include American Polarity Therapy Association, International Association of Health Care Practitioners, Reflexology Organization of Wisconsin. The Reflexology Research Project, Associated Bodyworkers, and Massage Professional. Wisconsin Natural Food Associates, and National Health Federation.
Dr. Gary Olson received his D.D.S. in 1976 from Marquette University School of Dentistry. He has been treating temporomandiular joint dysfunction, headaches, and related cervical pain for over 15 years. He was the Director of the Craniomandibular/Cervical Pain Center at St. Mary's Hospital in Milwaukee, Wisconsin, for two years from 1984-86. Dr. Olson maintained a private practice in Milwaukee from 1986-93 and then moved to Wisconsin Rapids where he currently practices. He provides treatment for TMD, headaches, and related cervical pain nonsurgically using conservative techniques recognized by American Dental Association and the Wisconsin Dental Association. Dr. Olson has lectured at national and international seminars and has published research findings completed with doctors at the Medical College of Wisconsin Milwaukee. He is a member of many pain-oriented professional organizations.
Collagen and muscle pathology in fibromyalgia
patients.
Gronemann ST, Ribel-Madsen S, Bartels EM,
Danneskiold-Samsoe B, Bliddal H.
The Parker Institute, Department of
Rheumatology, Frederiksberg Hospital, H:S University Hospital.
Rheumatology
(Oxford). 2003 Jul 16 @
OBJECTIVE: To measure collagen concentration and search for
muscle pathology in muscle non-tender-point areas from fibromyalgia (FM)
patients.
METHODS: Muscle biopsies were obtained from m. vastus lateralis of
27 carefully selected, female fibromyalgia patients, and from eight age-matched
female control subjects. Amino acids were determined by HPLC and electron
microscopy was performed.
RESULTS: The FM patients had lower hydroxyproline
and lower total concentration of the major amino acids of collagen than the
controls. No significant difference was seen in the concentration of the major
amino acids of myosin or of total protein. Electron microscopy showed no
significant differences between FM patients and controls although atrophied
muscle fibrils occurred in FM patients only, but frequencies were not
significantly different.
CONCLUSION: Fibromyalgia patients had a
significantly lower amount of intramuscular collagen. This may lower the
threshold for muscle micro-injury and thereby result in non-specific signs of
muscle pathology.
Collagen cross-links in fibromyalgia syndrome
Sprott H, Muller A, Heine H
Clinical Expermeriments
in Rheumatology 1998 Sep-Oct;16(5):626-7.
OBJECTIVE:
The acceptance of fibromyalgia as a disease entity and its definitive diagnosis
have been hampered by a dearth of knowledge concerning the underlying
pathophysiology of this disease and the lack of specific biochemical markers
applicable to its diagnosis. To determine whether abnormal collagen
metabolism is a characteristic of fibromyalgia, we have analyzed collagen
metabolites in the urine and serum of patients with fibromyalgia.
METHODS: The diagnosis of fibromyalgia was made according to the
American College of Rheumatology criteria. Urine and serum were collected under
standardized conditions from 39 patients and 55 age and sex matched controls.
Pyridinoline (Pyd) and deoxypyridinoline (Dpyd), which represent products
of lysyl oxidase mediated cross linking in collagen and are indicators of
connective tissue and bone degradation, respectively, were analyzed by
ion-paired and gradient HPLC method with fluorescence detection (HPLC). Levels
of hydroxypyroline (Hyp), a collagen turnover marker, were also measured. The
findings were related to creatinine levels and the Pyd/Dpyd ratio determined.
RESULTS: The Pyd/Dpyd ratios in the urine and serum and the Hyp in the
urine were significantly lower in patients with fibromyalgia than in healthy
controls.
CONCLUSION: Decreased levels of collagen cross linking may
contribute to remodeling of the extracellular matrix and collagen deposition
around the nerve fibers in fibromyalgia and contribute to the lower pain
threshold at the tender points. Analysis of altered collagen metabolism either
by histologic examination on biopsy or, preferably, by HPLC analysis of collagen
metabolites in urine or serum may aid in understanding more about the
pathogenesis of fibromyalgia.
Treatment of rheumatoid arthritis with oral type II collagen@. Results of a multicenter, double-blind, placebo-controlled trial.
Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, Fletcher MJ, Chasan-Taber S, Finger E, Morales A, Le CH, Trentham DE.
Beth Israel Deaconess Medical Center, Boston, Massachusetts 02215, USA.
Arthritis Rheum. 1998 Feb;41(2):290-7.
Objective
Oral administration of cartilage-derived type II collagen
(CII) has been shown to ameliorate arthritis in animal models of joint
inflammation, and preliminary studies have suggested that this novel therapy is
clinically beneficial and safe in patients with rheumatoid arthritis (RA). The
present study was undertaken to test the safety and efficacy of 4 different
dosages of orally administered CII in patients with RA.
Methods
Two hundred and seventy-four patients with active RA were
enrolled at 6 different sites and randomized to receive placebo or 1 of 4
dosages (20, 100, 500, or 2,500 microg/day) of oral CII for 24 weeks. Efficacy
parameters were assessed monthly. Cumulative response rates (percentage of
patients meeting the criteria for response at any time during the study) were
analyzed utilizing 3 sets of composite criteria: the Paulus criteria, the
American College of Rheumatology criteria for improvement in RA, and a
requirement for > or = 30 per cent reduction in both swollen and tender joint
counts.
Results
Eighty-three per cent of patients completed 24 weeks of
treatment. Numeric trends in favor of the 20 microg/day treatment group were
seen with all 3 cumulative composite measures. However, a statistically
significant increase (P = 0.035) in response rate for the 20 microg/day group
versus placebo was detected using only the Paulus criteria. The presence of
serum antibodies to CII at baseline was significantly associated with an
increased likelihood of responding to treatment. No treatment-related adverse
events were detected. The efficacy seen with the lowest dosage is consistent
with the findings of animal studies and with known mechanisms of oral tolerance
in which lower doses of orally administered autoantigens preferentially induce
disease-suppressing regulatory cells.
Conclusion
Positive effects were observed with CII at the lowest
dosage tested, and the presence of serum antibodies to CII at baseline may
predict response to therapy. No side effects were associated with this novel
therapeutic agent. Further controlled studies are required to assess the
efficacy of this treatment approach.
Effects of oral administration of type II collagen on rheumatoid
arthritis.
Trentham DE, Dynesius-Trentham RA, Orav EJ,
Combitchi D, Lorenzo C, Sewell KL, Hafler DA, Weiner HL.
Department of
Medicine, Beth Israel Hospital, Boston, MA.
Science. 1993 Sep
24;261(5129):1727-30.
Rheumatoid arthritis is an inflammatory synovial disease
thought to involve T cells reacting to an antigen within the joint. Type II
collagen is the major protein in articular cartilage and is a potential
autoantigen in this disease. Oral tolerization to autoantigens suppresses animal
models of T cell-mediated autoimmune disease, including two models of rheumatoid
arthritis. In this randomized, double-blind trial involving 60 patients with
severe, active rheumatoid arthritis, a decrease in the number of swollen joints
and tender joints occurred in subjects fed chicken type II collagen for 3 months
but not in those that received a placebo. Four patients in the collagen group
had complete remission of the disease. No side effects were evident. These data
demonstrate clinical efficacy of an oral tolerization approach for rheumatoid
arthritis.
Oral
type II collagen in the treatment of rheumatoid arthritis.
A six-month
double blind placebo-controlled study.
Cazzola M, Antivalle
M, Sarzi-Puttini P, Dell'Acqua D, Panni B, Caruso I.
Rheumatology
Unit, Azienda Ospedaliera Polo Universitario L. Sacco, Milan, Italy.
Clin
Exp Rheumatol. 2000 Sep-Oct;18(5):571-7.
OBJECTIVE: To evaluate the
efficacy of oral chicken type II collagen (CII) in the treatment of rheumatoid
arthritis (RA). METHODS: Sixty patients with clinically active RA of long
duration (mean 7.2 +/- 5.5 years) were treated for 6 months with oral chicken
CII at 0.25 mg/day (n = 31) or with placebo (n = 29) in a double-blind
randomized study. RESULTS: The response rate to treatment of the
collagen-treated group, based on the ACR 20% criteria, was higher than that of
the control group but this difference was not statistically significant at any
time. Intention-to-treat (ITT) analysis did not show statistically significant
improvement in any of the several secondary outcome measures over the 6 months
of the study in the collagen-treated patients in comparison with the
placebo-treated group. However, in 2 collagen-treated patients we observed a
clinical remission according to the criteria of the American Rheumatism
Association. CONCLUSION: Our study seems to show that the oral treatment of RA
patients with chicken CII is ineffective and results in only small and
inconsistent benefits. Furthermore, our results raise the possibility that in a
sub-group of patients oral collagen administration, usually considered devoid of
harmful effects, may actually induce disease flares.
Type II collagen serology: a guide to clinical responsiveness to
oral tolerance?
Gimsa U, Sieper J, Braun J, Mitchison
NA.
Deutsches Rheuma-Forschungs-Zentrum Berlin,
Germany.
Rheumatol Int. 1997;16(6):237-40.
A double-blind
placebo-controlled randomized trial of oral collagen type II (CII) treatment in
rheumatoid arthritis was completed in Berlin. Anti-CII antibody titres were
measured before and after the treatment. They showed that: (1) the titre prior
to treatment did not identify a responder subgroup, (2) the treatment reduced
CII antibody titres, but only in those patients making a clinical response and
(3) administration of 10 mg CII per day reduced the titre in these subsets more
effectively than 1 mg per day. Although the data are limited, they suggest that
a titre drop may be useful for identifying those patients who respond to this
form of treatment and that the drop may be a valid parameter for detecting the
impact of the treatment on the immune system.
Oral
type II collagen treatment in early rheumatoid arthritis. A double-blind,
placebo-controlled, randomized trial.
Sieper J, Kary S,
Sorensen H, Alten R, Eggens U, Huge W, Hiepe F, Kuhne A, Listing J, Ulbrich N,
Braun J, Zink A, Mitchison NA.
Deutsches Rheuma Forschungszentrum,
Berlin, Germany.
Arthritis Rheum. 1996 Jan;39(1):41-51.
OBJECTIVE. To investigate the efficacy of oral type II collagen in the
treatment of early rheumatoid arthritis (RA). |
METHODS. Ninety patients with
RA (disease duration < or = 3 years) were treated for 12 weeks with oral
bovine type II collagen at 1 mg/day (n = 30) or 10 mg/day (n = 30) or with
placebo (n = 30), in a double-blind randomized study.
RESULTS. There
were no significant difference between the 3 groups in terms of response to
treatment. However, we observed a higher prevalence of responders in the type II
collagen-treated groups: 7 responders in the 10-mg type II collagen group and 6
in the 1-mg group, versus 4 in the placebo group. Furthermore, 3 patients in the
10-mg type II collagen group and 1 patient in the 1-mg type II group, but no
patients in the placebo group, had very good response. A total of 14 patients
had to be withdrawn from the study: 2 because of side effects (nausea) and 12
because of lack of efficacy.
CONCLUSION. Only a minority of patients
responded to treatment with oral type II collagen. These results justify further
efforts to identify which patients will have good response to such therapy.
Effects of oral administration of type II collagen on
rheumatoid arthritis.
Trentham DE, Dynesius-Trentham RA,
Orav EJ, Combitchi D, Lorenzo C, Sewell KL, Hafler DA, Weiner
HL.
Department of Medicine, Beth Israel Hospital, Boston,
MA.
Science. 1993 Sep 24;261(5129):1727-30.@
Rheumatoid
arthritis is an inflammatory synovial disease thought to involve T cells
reacting to an antigen within the joint. Type II collagen is the major protein
in articular cartilage and is a potential autoantigen in this disease. Oral
tolerization to autoantigens suppresses animal models of T cell-mediated
autoimmune disease, including two models of rheumatoid arthritis. In this
randomized, double-blind trial involving 60 patients with severe, active
rheumatoid arthritis, a decrease in the number of swollen joints and tender
joints occurred in subjects fed chicken type II collagen for 3 months but not in
those that received a placebo. Four patients in the collagen group had complete
remission of the disease. No side effects were evident. These data demonstrate
clinical efficacy of an oral tolerization approach for rheumatoid
arthritis.
END